Company
Innovation, high technology, continuous evolution and inventiveness: these are the fundamental elements on which Nidek Technologies has always founded its success.

European Research and Development Center
MISSION
To design and develop innovative, technologically advanced equipment and software systems for diagnostic ophthalmology. Our efforts includes the clinical evaluation of such prototypes to test its efficacy on the field before to reach the market.
Our motto is: Anticipate the market needs to prepare the future.
HISTORY
 NIDEK was established in the summer of 1971 by a small group of 7 engineers headed by the founder Hideo Ozawa. Their spirit was explained by the 3 dreams they had in mind:
NIDEK was established in the summer of 1971 by a small group of 7 engineers headed by the founder Hideo Ozawa. Their spirit was explained by the 3 dreams they had in mind:

- “Invisible to Visible” (creating vision)
- “Visible to Recognition” (better recognition through vision)
- “Making Excellent Products for Vision” (leading the eye care market)
Today, with more than 1600 employees NIDEK expanded into the global business offering the widest range of products “for Eyes” in more than 100 countries.
Located in Padua, Italy, NIDEK Technologies is a NIDEK branch dedicated to high tech research and development.
Mainly focused on medical instrument and software intensive tools applied in Diagnostic Ophthalmology, NIDEK
Technologies operates in synergy with NIDEK Headquarters, Academic Institutions and International Engineers and Researchers from more than 20 years.
QUALITY
FDA 510 (K)
Under section 510(k) of the Act, a person who intends to introduce a device into commercial distribution is required to provide reasonable assurance of safety and effectiveness and to submit a premarket notification, or 510(k), to FDA.
A Premarket Notification is Required when:
- a new device is being introduced into the US market for the first time;
- the device is not exempt .
Food and Drug Administration has granted 510 (k) clearance for the following Nidek Technologies devices:
- 510(k) NO K012416: Confoscan Confocal Microscope
- 510(k) NO K023719: MP-1 Microperimeter
CE Mark
Nidek Technologies manages a full quality assurance system ensuring the conformity of products with the relevant general safety and performance requirements of the Medical Device Regulation 2017/745
Meaning of CE
CE Marking on a product is a declaration that the product complies with the essential requirements of the applicable legislations.
In addition:
1. CE Marking on a product indicates to governmental officials that the product may be legally placed on the market in their country.
2. CE Marking on a product ensures the free movement of the product within the EU single market.
3. CE Marking on a product permits the traceability and the withdrawal of the products potentially dangerous by the manufacturer and the vigilance authority.
To review the company's Quality Policy, follow this LINK.
INTELLECTUAL PROPERTY
Our knowledge is the most precious asset for us, this is the reason why we are continuously filing new patents.
Dedicated experts keep a deep relationship with the patents institutions to ensure its correct emission and validity over time.
PRODUCTS
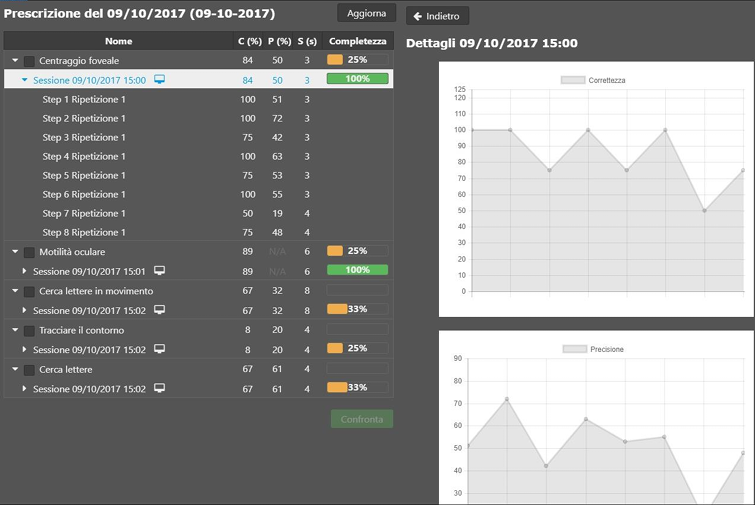 |
nLIFE EyeFitness 2.1.0 Basic UDI: ++B310NLIFEEFTT7 nLIFE EyeFitness is a medical device software (SaMD) that provides oculo-motor rehabilitation exercises to visually impaired patients via a tablet, notebook, or personal computer. The following indices are recorded during the execution of the exercise: correctness, precision, and speed. EyeFitness shows the results of the exercises performed by the patient in the dedicated nLIFE module. SHA256: 6a1681d5f8239d740759b406e0b3e9704dbf61c411d44ff3faba7a409eea4b2d |
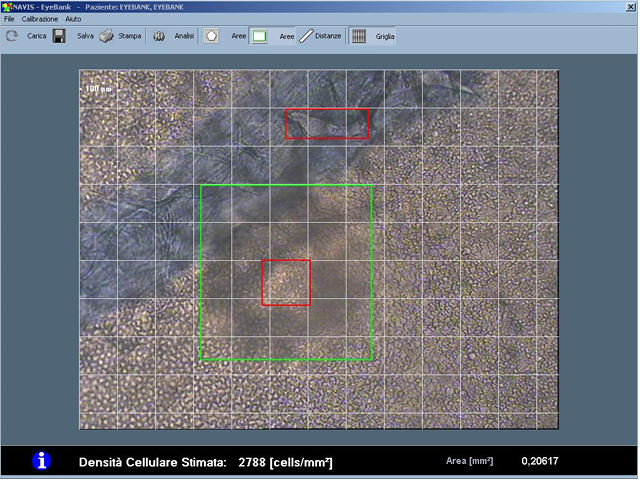 |
NAVIS EyeBank 3.7.3 The NAVIS EyeBank software automatically estimates endothelial cell density by processing images of explanted corneas, acquired with optical microscopes. It is therefore possible to acquire and store such images in a digital format and then process them and store and/or print the results of such analysis. Specifically, the Endothelial Cell Analysis software tool and the Cell Analysis EyeBank System tool support doctors to evaluate the physiological status of the explanted corneas giving information respectively on the morphological status and on the cell density of the considered endothelium SHA256: 594077f69d3dfb95983a6b8fb4aaedac0ecc5b2fff22e06ca13e17c582086869 |
Contact us for information
on our services